UNIT 1. BITS THAT MATTER
THE STRUCTURE OF MATTER
Description of Unit
This Unit deals with Matter- the material substance that constitute the observable universe and, together with energy, forms the basis of all objective phenomena.
At the most fundamental level, matter is composed of elementary particles known as quarks and leptons (the class of elementary particles that includes electrons). Quarks combine into protons and neutrons and, along with electrons, form atoms of the elements of the periodic table, such as hydrogen, oxygen, and iron. Atoms may combine further into molecules such as the water molecule, H2O. Large groups of atoms or molecules in turn form the bulk matter of everyday life.
We will discuss in depth the Periodic table of the elements- the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus.
When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. The initial discovery, which was made by Dmitry I. Mendeleyev in the mid-19th century, has been of inestimable value in the development of chemistry.
In this unit we will also continue to build on your knowledge of Matter from MVP 2. You know that matter is "anything that has mass and occupies space" (Chemicool.com, 2015), and you know how to measure it and what units to use. The building blocks of matter are known as "atoms", from the Greek word meaning "un-cuttable" (Skorucak, 2015).
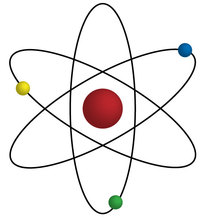
You have probably seen a picture like this before, which is one way to draw, model, an atom:
You have probably seen a picture like this before, which is one way to draw, model, an atom:
(Headberg, 2015)
But, how do we know what something looks like, if it is far too small to see?
But, how do we know what something looks like, if it is far too small to see?
EXTENSION-I can recommend a wonderful book- Bill Bryson's A Short History of Everything. " In Chapter 9-The Mighty Atom- Bryson introduces to his readers the history of the creation of the quantum theory, specifically surrounding the idea of atoms and the renowned scientists who are to be recognised for their participation. Mentions of Richard Feynman, John Dalton, and Ernest Rutherford accredit this piece of work, as all three eminent scientists gave their lives to the quantum realm, be it intentionally or unintentionally. What’s in an atom? Bryson asks.
Regarding the composition of an atom and a molecule, Bryson compares the relativity of size with various scales and analogies. One cubic centimetre of air (compared to about the size of a sugar cube) contains 45 billion billion molecules, a number so incredibly huge that it is difficult to grasp it in its entirety. Multiplying this by every single cubic centimetre in the universe, the number of molecules is virtually impossible to apprehend.
Bryson continues to provide the reader with intriguing explanations on the vigorous recycling of atoms, leading to an interesting point to consider:
The atoms in our air today very much has the possibility of once having belonged to Shakespeare, Rutherford, Albert Einstein, and the like. (Wow!)
More analogies reveal the minuteness of an atom’s size, with comparisons being made to paramecium (being larger than an atom despite standing only two microns wide) and the Empire State Building (an atom the thickness of a sheet of paper compared). Once Bryson has planted firmly the scale of atoms (one ten-millionth of a millimetre), we come to the realisation that atoms are three things: small, numerous, and practically indestructible.
MODELS OF THE ATOM
People have been thinking about what their world was made of for a long time. The first recorded discussions that matter is made of particles were over 2000 years ago when a Greek named Democretus suggested that matter is made of atoms, different materials contained different types of atoms and atoms could be "re-arranged" to make different substances. However, Aristotle did not agree with Democretus so people rejected the idea until Robert Boyle came along in 1661 (Splung.com, 2015).
Boyle gave us the modern definition of an element: "An element is a substance that can not be broken down into simpler substances but can form compounds with other elements" (Splung.com, 2015). Work through the following slides for an overview of how we have our current model of the atom:
Boyle gave us the modern definition of an element: "An element is a substance that can not be broken down into simpler substances but can form compounds with other elements" (Splung.com, 2015). Work through the following slides for an overview of how we have our current model of the atom:
HISTORY OF ATOMIC THEORY
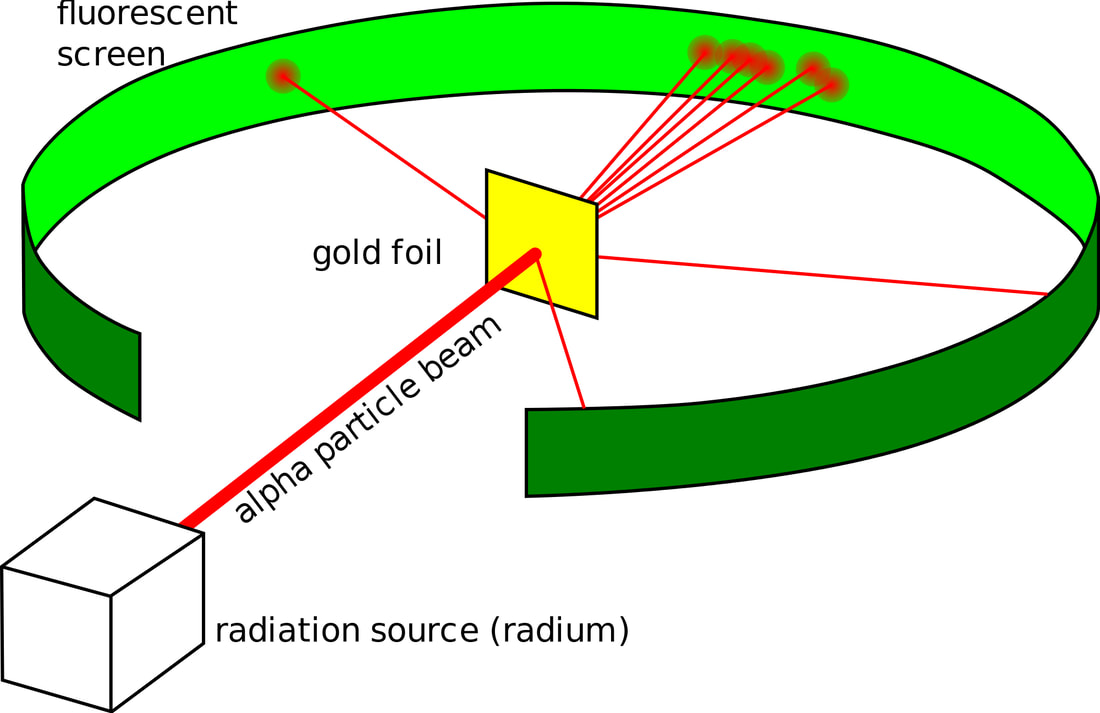
RUTHERFORD´S EXPERIMENT
QUESTIONS ABOUT ATOMIC MODELS
Task 4b: Answer the following questions in your NSD using full, clear sentences.
1. Can you name the four scientists credited with the atomic models?
2. Can you describe the differences between the original atomic model and the plum pudding model?
3. Can you find another instance where an apparent error or an accident has led to a new material or theory?
4. Can you explain why the nuclear model of the atom was not accepted until the electron shells model was presented?
5. What might have happened if Geiger and Marsden had used magnesium foil instead of gold?
6. Extension: How would you feel if you were J. J. Thomson and your atomic model was replaced in favour of the model of a student of one of your students?
Hint for question 5: Think about the sizes of the nuclei involved
1. Can you name the four scientists credited with the atomic models?
2. Can you describe the differences between the original atomic model and the plum pudding model?
3. Can you find another instance where an apparent error or an accident has led to a new material or theory?
4. Can you explain why the nuclear model of the atom was not accepted until the electron shells model was presented?
5. What might have happened if Geiger and Marsden had used magnesium foil instead of gold?
6. Extension: How would you feel if you were J. J. Thomson and your atomic model was replaced in favour of the model of a student of one of your students?
Hint for question 5: Think about the sizes of the nuclei involved



No comments:
Post a Comment